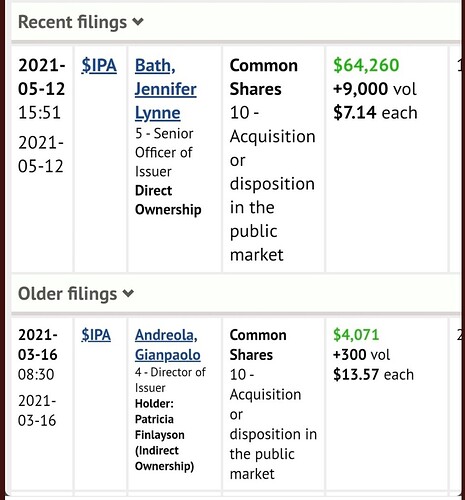
Est-ce que ça va y aller un jour avec IPA également ?!?
Bon plan pour stop la chute libre
Même si l’action ne monte pas ça donne de l’espoir pour un remède pour la covid ! 
ImmunoPrecise Moves SARS-CoV-2 PolyTope™ Cocktail Program’s Path Toward IND Filing
- Demonstrating what the Company believes to be the first reported in vivo synergistic effects of a multi-antibody (3+) combination treatment
- Dr. Ilse Roodink and Dr. Debby Kruijsen to host live webinar “Function First: Using complementary antibody platforms to fight Covid-19” today at 11:00 am Eastern Daylight Time
VICTORIA, British Columbia–(BUSINESS WIRE)-- ImmunoPrecise Antibodies Ltd. (the « Company » or « IPA ») (NASDAQ: IPA) (TSX venture: IPA) announces the completion of an efficacy-driven, preclinical study demonstrating further evidence of the in vivo efficacy of a four monoclonal antibody cocktail against non-overlapping epitopes, utilizing an optimized formulation and, for the first time, demonstrating therapeutic synergy of the cocktail components in vivo .
Highlights of the study include:
- Completed preclinical efficacy studies for its newly optimized 4-antibody cocktail (TATX-03b), showing strong in vivo therapeutic efficacy and synergy for the treatment and protection against SARS-CoV-2 infection
- The Company believes these are the first publicly reported in vivo synergistic effects of a multi-antibody (3+) combination treatment
- IPA’s optimized, 4-Ab cocktail (TATX-03b) shows high levels of efficacy in treating SARS-CoV-2 in the preclinical study
- Updated mutant binding data demonstrates continued in vitro resistance against novel variants of concern, including the recently described Californian and New York variants
- Company contracts ChemPartner Biologics (Shanghai) Co., Ltd. for optimized, stable CHO pool generation and cell bank generation for use in cGMP manufacturing
Live Webinar Today – “ “Function First: Using complementary antibody platforms to fight Covid-19” will be aired at 11:00 am Eastern Daylight Time (New York, GMT-04:00).
To learn how IPA’s PolyTope™ approach is designed to prevent the spread of novel variants, treat COVID-19, and prevent infection by SARS-CoV-2 and its variants, register for ImmunoPrecise’s webinar, to be held today , Thursday, May 20, 2021, hosted by Dr. Ilse Roodink and Dr. Debby Kruijsen.
…
Plus d’info dans le communiqué
Résumé de la présentation:
une question en deux parties sur le FasTrack FDA et la commercialisation.
Points saillants:
-Oui, IPA travail pour avoir le fastrack avec la FDA
-Conversations actives avec les organismes de réglementation (FDA) pour s’assurer que le package IND répond aux exigences minimales.
-7 mois pour avoir les anticorps prêts à la fabrication commercial. Mentionne quelle est contente avec cette timeline et ça pourra être optimalisée (Emergency Use Authorization)
-en ce qui concerne les essais cliniques « nous allons passer directement aux patients malades parce que nous avons un mode d’action différent » (commencer avec des patients atteintes du COVID signifie sauter la Phase I tradionnelle) «Et IPA prévoit faire une phase continue 1, 2, 3 » pour accélérer la commercialisation
Un indice sur un éventuel partenaire ?
rien mentionné dans la presentation mais le video de IPA cette semaine semble etre dirigé vers un partenaire.
ImmunoPrecise Names Dr. Dion Neame to Strategic Advisory Board
IMMUNOPRECISE ANTIBODIES LTD. (the « Company » or « IPA ») (NASDAQ: IPA) (TSX VENTURE: IPA) a leader in full-service, therapeutic antibody discovery and development, today announced that Dr. Dion Neame has joined the company’s Strategic Advisory Board (SAB). Dr. Neame joins at a significant time, as the company continues to focus its energy on building essential partnerships and expanding its rapidly growing share of the market. He will play a key role in supporting the strategic and scientific prioritization of partnerships and stakeholder communications.
Dr. Neame is a clinical medicine and vaccine expert, focused on medical strategies for various indications and early pipeline products, as well as external medical and scientific stakeholder engagements.
With over 15 years’ experience in large pharma, Dr. Neame has held many impressive leadership roles, including North American Senior Medical Expert and Country Medical Lead, Canada. He also has extensive experience as a medical professional, including pediatric medicine at notable hospitals such as McMaster Children’s Hospital and as Chief of Pediatrics Joseph Brant Hospital in Ontario, Canada.
“The addition of Dr. Neame to our SAB is instrumental in helping to bring IPA to the next level of growth and strategic execution,” noted Dr. Jennifer Bath, ImmunoPrecise’s CEO. “His scientific expertise in translational research, medical governance and compliance standards, combined with his expertise in stakeholder engagement, will play an important role as we execute on our strategic plans. It is wonderful to have someone with Dr. Neame’s breadth and depth of experience - from both a business and scientific perspective - to join us at this exciting moment for IPA.”
Dr. Neame, the 2020 recipient of the Innovative Medicines Respected Partner Award, who also serves as a consulting pediatrician, has concurrently held the position of Assistant Clinical Professor at McMaster University (Hamilton, Ontario) in the Department of Pediatrics for over 20 years. Throughout his career, Dr. Neame has contributed to numerous vaccine research programs and has been instrumental in establishing the medical, scientific and health economic rationale for 16 new vaccination programs in provinces and territories across Canada.
“I look forward to joining ImmunoPrecise’s Strategic Advisory Board,” stated Dr. Neame. “We are living in turbulent times and the need for novel therapeutic antibodies with broad epitope coverage has never been more important, to compliment COVID-19 vaccines to control SARS-CoV-2 variants of concern. IPA is a world-class Contract Research Organization (CRO), rich in science and housing cutting-edge antibody platform technologies. The Company’s custom approaches enable multiple antibody pathways, providing agile and adaptable discoveries to match agile and adaptable infectious microorganisms, as showcased in their work targeting SARS-CoV-2.”
Aucune mention à propos de Sanofi cependant…
Last week, Moderna held its fourth annual Science Day. In this virtual event for investors, the company laid out its research and development plans. But the biotech’s top scientists also sounded an alarm reminiscent of Paul Revere’s famous call that the British were coming. In this case, though, Moderna’s warning was that new COVID-19 waves are coming.
Moderna chief scientific officer Melissa Moore stated, « As the virus spreads, it is rapidly mutating. » She added, « Some of these new viral strains appear to be even more transmissible than the original strain. »
The good news is that vaccines from Moderna and Pfizer, in particular, have been highly effective so far. The bad news is, in Moore’s words, « We already know that some of these new strains are less susceptible to neutralization by our current vaccine. »
Moderna’s researchers have found lower neutralizing antibody levels against several key coronavirus variants to be of concern, which suggests a risk of reduced duration of protection against infection. Because some of these variants are more transmissible, Moderna thinks that « new epidemic waves are undoubtedly on the way. »
Preclinical Data from ImmunoPrecise’s Drug Pipeline to be Presented During the BIO International Partnering Event
Business Wire
Publishing date:
Jun 15, 2021 • 30 minutes ago • 5 minute read • Join the conversation
Article content
VICTORIA, British Columbia — IMMUNOPRECISE ANTIBODIES LTD (the “Company” or “IPA”) (NASDAQ: IPA) (TSX VENTURE: IPA) today announced the results from in vitro characterizations investigating TATX-21, a novel potential first-in-class antibody for Atherosclerosis Cardiovascular Disease (ACVD), will be presented in partnering meetings at the 2021 BIO International Convention, June 14-18, 2021.
Data will be presented on investigational antibodies known as TATX-21, a diverse pool of antibodies (cross-)reactive to human and murine undisclosed paralogous targets. The antibodies, isolated via IPA’s single B cell SelectTMtechnology, were discovered using rabbit B cells as the source of novel antibodies, a host cell utilized by ImmunoPrecise with the aim to maximize the diversity and function of the resulting lead candidates. The lead candidate pool of 25 sequence-unique antibodies has been advanced for further testing to determine their potential to prevent low-density lipids (LDL) uptake, functional tests that are intended to shine light on the antibodies’ potential to prevent and treat Atherosclerosis Cardiovascular Disease (ACVD).
The Company noted that they are screening the lead candidate pool for the ability to block the interaction of the target of interest with LDL but also for their ability to stimulate the target thereby potentially activating a down-stream signaling cascade, which may, based on published literature, provide a novel treatment approach for diabetic retinopathy.
“These data, which will be presented at IPA’s BIO Digital partnering meetings, further our understanding of these novel antibodies and their mechanisms of action and inform our thinking regarding potential program opportunities to treat two very different but serious indications,” said Jennifer L Bath, Ph.D., CEO and President at ImmunoPrecise Antibodies. “This is an exciting year for our internal therapeutics pipeline, as results from a number of research and development programs continue to advance.”
About B cell Select™
IPA’s B cell Select™ platform enables the interrogation of a greater diversity of an antibody repertoire. By interrogating isolated B cells, IPA can analyze full organism repertoires with very little manipulation. This proprietary platform is species independent allowing for the generation of antibodies from samples not possible using other methods. B cell Select has the potential to develop antibodies from any species (including humans) as well as from any tissue. As the platform explores the entire antibody repertoire, it provides the opportunity to develop antibodies for anything that is possible in an animal’s immune repertoire including any protein class, complex therapeutic targets, post-translational modifications, and small molecules.
The B cell Select platform enables the interrogation of up to ten million blood cells to generate native monoclonal antibodies from immunized animals that specifically target an antigen. The B cell Select process takes place early in the antibody development process allowing for the rapid selection of top candidates, which IPA believes drastically increases the success rate of antibody discovery. The platform also harnesses the power of the immune system to generate natural pairing of the antibodies produced by selected B cells.