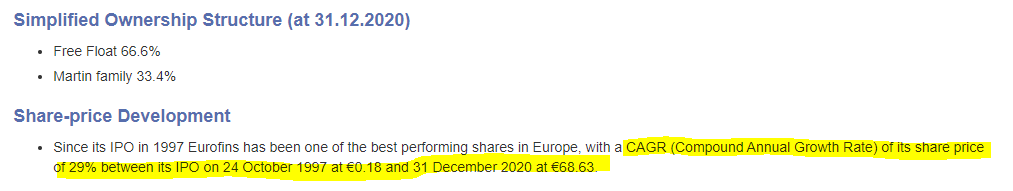
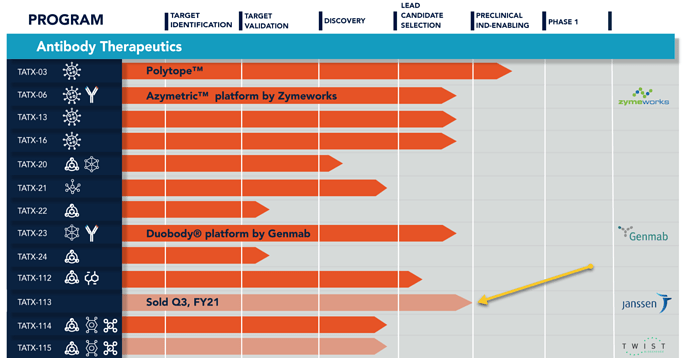
Treatment with TATX-03a substantially reduced bronchitis and tracheitis severity in preclinical studies
-
New histopathology data show that the significantly reduced viral load observed after prophylactic and therapeutic treatment with TATX-03a is accompanied by minimal lung inflammation in SARS-CoV-2 challenged hamsters.
-
Antibody sequences modified to facilitate manufacturing and clinical studies. The ability of the modified antibodies to bind target was not altered by the optimization.
-
Unaffected binding profiles of optimized TATX-03 cocktail antibodies released, which are anticipated to increase clinical suitability.
-
Dr. Ilse Roodink and Dr. Debby Kruijsen to host live webinar Thursday, June 17th, 2021, at 11:00 a.m. ET (8:00 a.m. PT)
June 17, 2021 08:28 AM Eastern Daylight Time
VICTORIA, British Columbia–(BUSINESS WIRE)–IMMUNOPRECISE ANTIBODIES LTD (the « Company » or « IPA ») (NASDAQ: IPA) (TSX VENTURE: IPA) today announced additional results from its in vivo hamster challenge efficacy study of TATX-03a PolyTope™ Therapy, a four monoclonal antibody cocktail being developed for the potential prevention and treatment of SARS-CoV-2, demonstrating reduced bronchitis and tracheitis inflammation severity. The Company concurrently announced optimization results of TATX-03 cocktail components designed to improve clinical suitability of their PolyTope therapy.
“These observations indicate that the previously reported data of reduced replication-competent viral titers following TATX-03 treatment also translate to decreased disease severity in the hamster challenge model, strengthening the potential of our PolyTope therapy for prevention and treatment of COVID.”
Histopathology data of efficacy study in SARS-CoV-2 challenged hamsters:
The primary endpoint evaluated in the preclinical study previously announced on February 19, 2021, measured viral load as the main indicator of therapeutic efficacy. The recently completed histopathology analyses of the tissues harvested from this same study demonstrate that prophylactic and therapeutic treatment using TATX-03a on SARS-CoV-2 challenged animals also reduced inflammation severity of the main conducting airway tissues compared to study control animals. In addition, only mild inflammation of the trachea was observed in animals treated with TATX-03a post-challenge.
“It is very encouraging that, in addition to meeting the study endpoint criteria evaluating the impact of our therapy on viral load, treatment with TATX-03a also reduced bronchitis and tracheitis severity” stated Dr. Ilse Roodink, IPA’s Global Program Director for COVID Research. “These observations indicate that the previously reported data of reduced replication-competent viral titers following TATX-03 treatment also translate to decreased disease severity in the hamster challenge model, strengthening the potential of our PolyTope therapy for prevention and treatment of COVID.”
Light molecular optimization of two cocktail components intended to promote potential clinical success:
In parallel to in vivo efficacy evaluation, the Company assessed the developability profiles of the individual components of TATX-03. Based on this assessment, the Company applied light molecular optimization to selected antibodies with the goal to minimize manufacturing liabilities and promote potential clinical success of their PolyTope SARS-CoV-2 therapy. The optimization focused on removal of sites in the antigen binding fragment that might not be fully processed during clinical product development, and which had the potential to reduce clinical manufacturing yields. Optimization also addressed replacing non-germline residues, a process intended to reduce immunogenicity risks. Reactivity screening performed by the Company confirmed that the binding profiles of the modified antibodies were not affected during product optimization. The Company anticipates that these optimization efforts positively impact clinical suitability of TATX-03. Stable pool generation of the optimized leads to source toxicology/toxicokinetic studies and to generate cGMP cell banking is currently ongoing at ChemPartner Biologics (Shanghai) Co., Ltd.
Simultaneous to the molecular optimization described herein, the Company continues to plan for IND submission of TATX-03 to pursue human clinical studies evaluating the potential of the cocktail to protect and/or treat COVID-19 patients.
To learn how IPA’s PolyTope™ approach and the data obtained to date about its potential to prevent and treat by SARS-CoV-2 and tested variants of concern, register for ImmunoPrecise’s webinar, to be held today, Thursday, June 17, 2021, at 11:00 a.m. ET hosted by Dr. Ilse Roodink and Dr. Debby Kruijsen.
Click HERE to Register