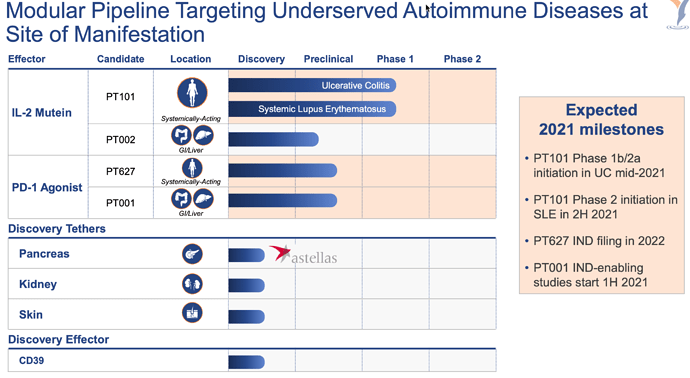
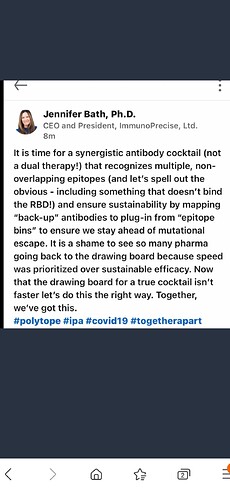
Nouvelle publication (Feb 22) de la FDA sur le « fast track » des therapies d’anticorps contre les nouvelles variantes du virus COVID.
Tres interressant parce que cette nouvelle initiative « Emergency Use » est pour la prochaine generation de therapy contre les variantes et le program semble etre plus vite que le program precendant de « Warp ». Quelle sont vos pensés?
• Given the serious concerns raised by the emergence of SARS-CoV-2 variants, FDA
intends to leverage its emergency authorities under section 564 of the FD&C Act, when
appropriate, to foster the development and availability of therapeutics for use during the
current public health emergency. When scientifically supported, FDA will streamline the
data necessary to support the development of monoclonal antibody products targeting
SARS-CoV-2 and also expedite the review of these data.
• Given the dynamic public health situation created by changes in the prevalence of new
variants over time, FDA’s streamlined approach will depend on its current assessment of
benefit and risk for the intended use and population, the monoclonal antibody’s expected
coverage of important emerging variants, as well as FDA’s then-current understanding of
SARS-CoV-2.
• FDA strongly recommends that individual monoclonal antibody products be developed
with the expectation that they will be combined with one or more monoclonal antibody
products that bind to different epitopes5 to minimize the risk of losing activity against
emergent variants. FDA encourages collaborations between sponsors of individual
monoclonal antibody products to address this unmet medical need.
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-monoclonal-antibody-products-targeting-sars-cov-2-including-addressing-impact-emerging