Ils ont plus de $$$ ainsi qu’une plateforme Transgénique mais quand même l’écart de valorisation pour la technologie est incroyablement alléchante en faveur de IPA
Je crois que le gap de valorisation est parce que AbCellera a les mots “intelligence artificielle” dans sa description 
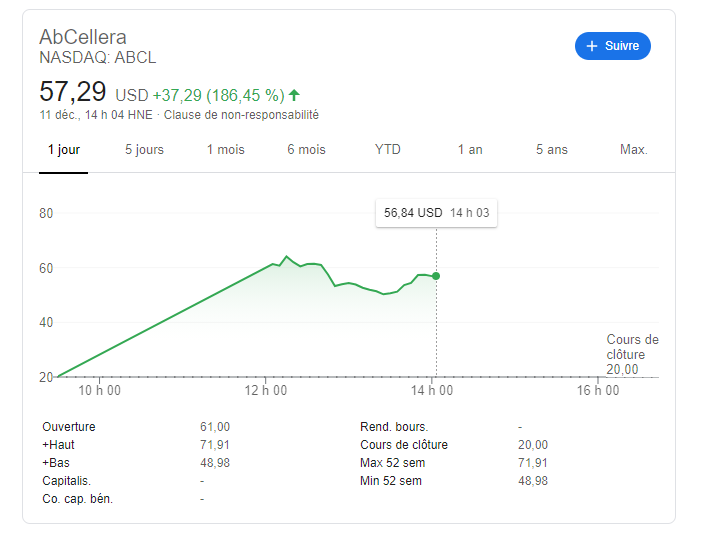
Au contraire, AbCellera a eu une croissance de 200% YoY alors qu’IPA a eu une croissance de 28% sur la dernière année fiscale et de 39% YoY pour les 3 derniers mois.
À moins que vous soyez confiants que les revenus d’IPA vont exploser et tripler (et devenir profitable), AbCellera est dans une ligue différente.
Pas qui justifie $4B en valo à moins d’inclure une valeur stratégique de leur tech. Le cas échéant IPA est en excellente position
Jennifer a dit lors de l’entrevue (si je me rappelle bien c’est celle avec smallcapdiscoveries), qu’elle s’attend à ce que les revenus provenant de Talem dépasse rapidement les revenus actuels
Pre-IPO: Everything You Need To Know About AbCellera (Part I)
La date di IPO toujours pas connue ou c’est moi qui ne l’a pas vu?
Les autorités sanitaires britanniques ont déconseillé mercredi d’inoculer le vaccin de Pfizer et BioNTech contre le nouveau coronavirus aux personnes ayant eu dans le passé d’«importantes réactions allergiques», deux personnes ayant mal réagi aux premières injections.
Les gouvernements sont sous pressions avec le confinement. Ils veulent des solutions rapides mais cela va engendrer des risques assurément.
Voici la présentation d’Abcellera https://www.retailroadshow.com/
"Still, the Mayo Clinic’s Poland says that vaccine makers that can innovate have the most chance of winning long term, regardless of their current status, as most of the world still awaits protection. “The idea that the window is closing for new coronavirus vaccines is completely wrong and counterintuitive,” he says. “The window is closing for manufacturers who think they can do things in the same old ways.”
ImmunoPrecise Collaboration Enables Preclinical Manufacturing of Lead Antibodies Targeting SARS-CoV-2
VICTORIA, BC, Dec. 11, 2020 /CNW/ - IMMUNOPRECISE ANTIBODIES LTD. (the « Company » or « IPA ») (TSXV: IPA) (OTCQB: IPATD) (FSE: TQB2), a leader in full-service, therapeutic antibody discovery and development, today announced that it has entered into a research collaboration with the National Research Council of Canada’s(NRC) Human Health Therapeutics (HHT) Research Centre to develop its neutralizing PolyTope™ antibodies against SARS-CoV-2.
IPA is also considering acquiring a license for the NRC CHO cell-based rapid expression platform to be used in pre-clinical and clinical manufacturing of their lead monoclonal antibodies. Researchers from the NRC’s HHT Research Centre will deploy the CHO-based expression platform to accelerate the identification and development of IPA’s lead antibody candidates.
The NRC is supporting this research through the Pandemic Response Challenge Program (PRCP) and the Industrial Research Assistance Program (IRAP). The project is also in collaboration with Zymeworks Inc. (NYSE: ZYME) for the design and development of IPA’s lead antibody candidates. ImmunoPrecise identified antibodies directed against the SARS-CoV-2 virus spike protein during the summer of 2020 by screening tens of thousands of antibodies from multiple sources and converging upon a panel of candidates that showed in vitro functional activity and synergistic effects in pseudovirus-based neutralization assays. Top neutralizing antibodies have been progressed for preclinical testing in the PolyTope™ Therapy program.
IPA considers the NRC to be a partner of choice to develop and manufacture their antibody therapeutics, as the NRC is uniquely positioned in Canada to address the various facets of this project having integrated, multi-disciplinary biologics research facilities based in Montréal and Ottawa.
« IPA is pleased to contribute to this Canadian endeavor with the NRC and Zymeworks, » stated Jennifer Bath, CEO of ImmunoPrecise. « IPA is proud to embark on its first collaboration with the NRC, as we continue to establish ourselves as a key player in the Canadian life science ecosystem and in the current pandemic. We believe this partnership between the NRC (HHT, PRCP and IRAP) and Zymeworks to have tremendous potential to generate best-in-class SARS-CoV-2 therapeutics within this expert and motivated team environment in Canada. »
Aujourd’hui Certara (CERT) qui est une société de services en bio informatique a vu son prix d’IPO passé de 23 $ a 38$. Le marché est très réceptif pour les IPO , même si ca demande beaucoup de paperasse , ca vaut l’effort de financer et valoriser son entreprise en même temps,
C’est peut-être ça le délai… ils ont peut-être changé leur stratégie 
ImmunoPrecise and LiteVax Advance SARS-CoV-2 Vaccine Candidate
VICTORIA, BC, Dec. 14, 2020 /CNW/ - IMMUNOPRECISE ANTIBODIES LTD. (« IPA ») (TSXV: IPA) (IPATD: IPA) (FSE: TQB), a leader in full-service, therapeutic antibody discovery and development and LiteVax BV (Oss, the Netherlands), today announced the nomination of a lead vaccine for further (pre-) clinical evaluation and development based on results from their collaborative preclinical immunogenicity study. IPA and LiteVax selected the vaccine candidate following an assessment of the immunogenicity profiles of multiple SARS-CoV-2 vaccine candidates, each having an empirically designed, single SARS-CoV-2 spike protein segment, in non-rodent species. Using IPA’s extensive data sets, candidates were screened and optimized to maximize the inclusion of functional, antigenic, epitopes while simultaneously minimizing the total foreign epitope exposure, thereby potentially reducing long-term, negative side effects.
Immunization of swine with a low dose of the selected candidate resulted in significant serum reactivities towards the SARS-CoV-2 spike protein segment. Furthermore, select formulations were adjuvanted with LiteVax’s novel class of carbohydrate derivative-based adjuvant to evaluate the potential benefit of co-formulation. When compared to non-adjuvant formulations, the combination with LiteVax’s adjuvant induced substantially higher immune responses. No adverse effects were observed for any of the treatment groups. This study was held at IRTA, (Catalonia, Spain) and supported by TRANSVAC2, a vaccine research and development (R&D) infrastructure that aims to accelerate the development of safe, effective and affordable vaccines (EC-funded project, grant agreement N° 730964).
« We are inspired by the positive results from our initial preclinical studies evaluating the potential of PolyTope therapies as single-low-dose vaccines for diseases such as SARS-Cov-2. In these studies, our newly selected vaccine formulation demonstrated significant antibody responses towards the full SARS-Cov-2 spike timer following a single injection, an effect that was enhanced through co-formulation with LiteVax’s adjuvant, » stated Ilse Roodink, Global Project Lead for the Company’s Coronavirus programs. "By leveraging a data-driven approach to the design of the SARS-CoV-2 spike protein, we are able to minimize unnecessary exposure while optimizing efficacy and are confident that we are nominating the best possible candidate from this study for continued development. We look forward to progressing this partnership and to continuing to learn about the potential of this unique collaboration to provide a safe, effective, vaccine for at-need patients.
Viral neutralization potency screening of the induced immune responses is scheduled for January. IPA and LiteVax also anticipate initiating additional, parallel preclinical studies to evaluate the durability and efficacy of immune responses in large non-rodents in more detail, as well as in a SARS-CoV-2 Syrian hamster model.
Si je ne me trompe pas, ipa se transige maintenant sur le nasdaq.
Pas encore