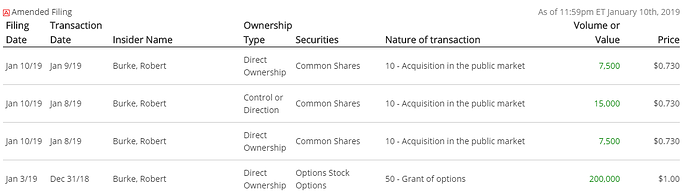
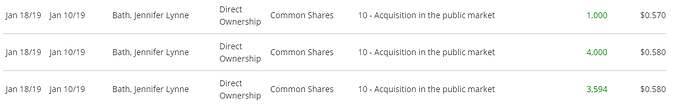
Même après l’octroi d’options, Robert Burke ajoute à sa position sur le marché :
C’est modeste pour l’instant mais je pense que plusieurs investisseurs seront contents de voir la CEO acheter sur le marché :
Présentation au Noble Healthcare Conference en Floride cette semaine
Le lien pour la conférence https://www.nobleconference.com/NobleCon15/about.htm
Lien direct … question de vous sauvez du temps 
Présentations pour investisseurs
VICTORIA, Feb. 5, 2019 /CNW/ - IMMUNOPRECISE ANTIBODIES LTD. (the “Company” or “IPA”) (TSX VENTURE: IPA) (OTC PINK: IPATF) has announced the purchase of its second state-of-the-art Intellicyt® iQue Screener PLUS for high-throughput cell-based screening.
The demand for accelerated development of next-generation antibodies that recognize evermore challenging targets and have improved activities has prompted IPA to purchase further instrumentation to evaluate more antibody characteristics in a multiplexed, high-throughput way. Traditional antibody screening tools report on binding one target at a time, which makes the discovery process time consuming and can require large amounts of target protein. The Intellicyt iQue allows IPA scientists to significantly reduce lead discovery time while conserving valuable samples and reducing reagent cost.
“The decision to acquire a second Intellicyt iQue [now for North America] will further support IPA’s strategic vision of continued growth and expansion in providing end-to-end therapeutic antibody discovery services to a rapidly expanding list of pharma and biotech clients.” said Jennifer Bath, CEO and President of ImmunoPrecise Antibodies.
The iQue Screener PLUS can analyze antibodies developed from all of IPA’s antibody discovery platforms, hybridoma, B cell select, and phage display, on cells or beads in suspension. The early stage screening of antibodies on cell surface targets provides more predictable data and enables the selection of lead candidate drugs earlier and faster. In addition, the iQue provides early data on the activities of lead drugs to improve the probability for downstream successes.
About ImmunoPrecise Antibodies Ltd.
ImmunoPrecise is a full-service, therapeutic and diagnostic antibody discovery company focused on the next generation of antibody discovery, to deliver the most relevant antibodies, in a shorter period of time, with the highest probability of succeeding through clinical validation.
ImmunoPrecise Antibodies Ltd operates from three state-of-the-art laboratory facilities. ImmunoPrecise Antibodies (Canada) is located in Victoria, British Columbia, while U-Protein Express B.V., and ModiQuest Research B.V. are located in Utrecht and Oss, Netherlands, respectively. The Company operates globally to offer antibody services from target analysis to pre-clinical studies.
The services offered to clients include antibody discovery for a broad spectrum of antigens, including challenging targets. Among these services, the Company offers antibody development directly from B-cells (B-Cell Select), through phage display, and by hybridoma production. The Company also provides a broad range of supporting services including immunologically-based assays, recombinant protein manufacturing, humanization, optimization, stable cell line development, and advanced solutions to challenges faced by clients in antibody-related research and development. The antibodies produced by ImmunoPrecise target a wide variety of therapeutic, diagnostic and research applications.
Second US Office for ImmunoPrecise Antibodies, Ltd. in Cambridge, Massachusetts
Canada NewsWire
VICTORIA, Feb. 14, 2019
VICTORIA, Feb. 14, 2019 /CNW/ - IMMUNOPRECISE ANTIBODIES LTD. (the « Company » or « IPA ») (TSX VENTURE: IPA) (OTC PINK: IPATF) has announced that they have opened a new subsidiary office in Cambridge, Massachusetts.

ImmunoPrecise Antibodies Ltd is a Canadian contract research organization providing a range of services, including antibody discovery, manufacturing, optimization, engineering and pre-clinical studies.
« With production sites in Canada and the Netherlands, and the executive headquarters in the North Dakota, establishing a base in the Boston / Cambridge area, a powerhouse for biotech and pharma, is a strategic next step for IPA. We already have a strong base of both European and US clients, and this move will help us enhance and grow our Boston area business and relationships », said Jennifer Bath, CEO of IPA.
The new office is located at One Broadway, 14th floor, Cambridge, MA 02142 and will initially be staffed for sales and business development initiatives.
« There is a tremendous demand for pioneering antibody discovery and development services in the greater Boston area, the largest US biotech hub, and we are excited to provide day-to-day access to our clients and prospective clients as we continue to offer an unparalleled continuum of antibody discovery and downstream technologies, » stated Dr. Jennifer Bath, CEO and President of IPA.
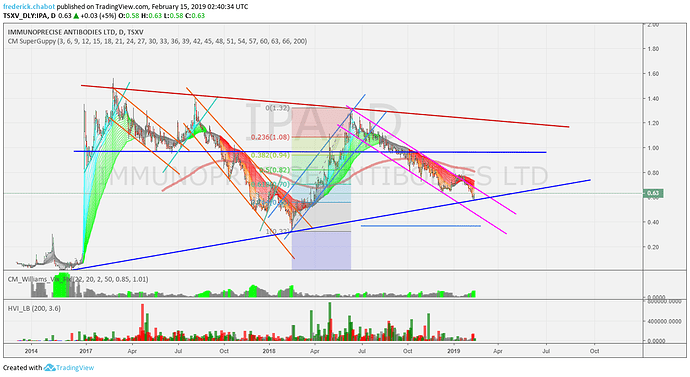
Je pense qu’avec la session d’aujourd’hui IPA vient de concéder une partie de son mystère dans sa structure. Voyons voir avec les prochaines séances si le Drapeau se confirme.
ImmunoPrecise Antibodies Collaborates with FIND to Help Advance New TB Diagnostic Test
VICTORIA, Feb. 19, 2019 /CNW/ - IMMUNOPRECISE ANTIBODIES LTD. (the « Company » or « IPA ») (TSX VENTURE: IPA) (OTC PINK: IPATF) announces the publication of the results of a research collaboration project with the Foundation for Innovative New Diagnostics (FIND) where IPA developed rabbit monoclonal antibodies (mAbs) for assessment in FIND’s sensitive new immunoassay diagnostic test for tuberculosis (TB). IPA developed rabbit monoclonal antibodies, using its B cell Select platform, that recognize lipoarabinomannan (LAM), a lipopolysaccharide target and biomarker for TB that can be excreted in the urine of individuals with the disease. Two of these antibodies, when combined with others to form capture-detection pairs and a sensitive chemical detection reagent, were highly specific for LAM and sensitive enough to detect LAM in urine samples.

FIND believes that early diagnosis is crucial for achieving the goal of decreasing the estimated 1.7 million deaths caused by tuberculosis each year. Developing an affordable point-of-care diagnostic that is sensitive enough to accurately detect the disease in easily-obtainable samples is a challenging, but critical part of achieving that goal.
IPA’s propriety B cell Select platform enables antibody discovery directly from the B cells derived from tissues of many species of animal, including rabbits, while maintaining the antibodies’ native heavy and light chain pairings. « Organizations that develop immunoassays as diagnostic tests depend on the discovery of sensitive and specific mAbs. These reagents form the foundation for dependable diagnostics that could ultimately increase the early diagnosis and thus decrease the effects of many diseases worldwide, » said Jennifer Bath, President and CEO of ImmunoPrecise Antibodies.
« We are pleased with the outcome of this successful collaboration, » said Claudia Denkinger, Head of Tuberculosis program at FIND. « Just 3 months after immunization we were able to use the high-affinity rabbit monoclonal antibody developed by ImmunoPrecise in a sensitive assay to detect the tuberculosis antigen. The antibody was highly specific with excellent affinities. »
The scientific publication titled: A Novel Sensitive Immunoassay Targeting the 5-Methylthio-d-Xylofuranose–Lipoarabinomannan Epitope Meets the WHO’s Performance Target for Tuberculosis Diagnosis, was published in the Journal of Clinical Microbiology 2018 Dec; 56(12)
About ImmunoPrecise Antibodies Ltd.
ImmunoPrecise is a full-service, therapeutic and diagnostic antibody discovery company focused on the next generation of antibody discovery, to deliver the most relevant antibodies, in a shorter period of time, with the highest probability of succeeding through clinical validation.
ImmunoPrecise Antibodies Ltd operates from three state-of-the-art laboratory facilities. ImmunoPrecise Antibodies (Canada) is located in Victoria, British Columbia, while U-Protein Express B.V., and ModiQuest Research B.V. are located in Utrecht and Oss, Netherlands, respectively. The Company operates globally to offer antibody services from target analysis to pre-clinical studies.
The services offered to clients include antibody discovery for a broad spectrum of antigens, including challenging targets. Among these services, the Company offers antibody development directly from B-cells (B-Cell Select), through phage display, and by hybridoma production. The Company also provides a broad range of supporting services including immunologically-based assays, recombinant protein manufacturing, humanization, optimization, stable cell line development, and advanced solutions to challenges faced by clients in antibody-related research and development. The antibodies produced by ImmunoPrecise target a wide variety of therapeutic, diagnostic and research applications.
About FIND:
FIND (Foundation for Innovative New Diagnostics) is an international non-profit organization that enables the development and delivery of much-needed diagnostic tests for poverty-related diseases, including tuberculosis, malaria, HIV/AIDS, sleeping sickness, hepatitis C, leishmaniasis, Chagas disease, Buruli ulcer, febrile illnesses and infectious diseases with outbreak potential, such as Ebola.
FIND acts as a bridge between experts in technology development, policy and clinical care, reducing barriers to innovation and effective implementation of diagnostic solutions in low- and middle-income countries.
Le contrat a une valeur approximative de $5M USD pour l’instant. Ce secteur est en changement rapide et ce genre de partenariat va devenir une norme.
ImmunoPrecise Antibodies Receives Subcontract to Produce Rabbit Monoclonal Antibodies
VICTORIA, Feb. 21, 2019 /CNW/ - IMMUNOPRECISE ANTIBODIES LTD. (the « Company » or « IPA ») (TSX VENTURE: IPA) (OTC PINK: IPATF) has received a five-year Indefinite Delivery/Indefinite Quantity (ID/IQ) subcontract from Leidos Biomedical Research, Inc., which currently operates the Frederick National Laboratory for Cancer Research for the National Cancer Institute (NCI). Under the subcontract, IPA will discover and produce rabbit monoclonal antibodies using its proprietary B-Cell Select platform.

The rabbit monoclonal antibodies discovered and produced under this subcontract will be used in the key applications of immunohistochemistry and immuno-Multiple Reaction Monitoring (immuno-MRM). The antibodies will be generated for NCI’s Antibody Characterization Program. Immuno-MRM is a leading-edge targeted mass spectrometry technique that relies on antibodies to enrich peptides from complex biological samples that are then quantified by mass spectrometry. Rabbit monoclonal antibodies are highly sought after for this purpose because they exhibit high specificity and bind their target with high affinity. Immuno-MRM is being used to discover novel biomarkers and develop a new generation of clinical diagnostic tests that could lead to improved cancer detection, diagnosis, and treatment.
IPA’s B-Cell Select platform enables rabbit monoclonal antibody discovery directly from B-cells through recombinant DNA cloning and expression of antibody gene sequences. This process allows for large panels of antibodies to be screened for functionality via multiple methods to pick the most relevant leads for the final application and can be applied to discover antibodies from several species for diagnostics and therapeutics.
« We are very pleased to work with Leidos Biomedical to support the world-class cancer research funded by NCI and NIH globally. We look forward to applying IPA’s experience and expertise in developing rabbit monoclonal antibodies to the challenging projects being advanced through Leidos » said Deanna Dryhurst, CSO of IPA.
About Frederick National Laboratory for Cancer Research
The Frederick National Laboratory for Cancer Research is dedicated to improving human health through discovery and innovation in the biomedical sciences, focusing on cancer, AIDS, and rapid response to emerging infectious diseases. Frederick National Laboratory is operated by Leidos Biomedical Research, Inc. for the National Cancer Institute.
About ImmunoPrecise Antibodies Ltd.
ImmunoPrecise is a full-service, therapeutic antibody discovery company focused on the next generation of antibody discovery, to deliver the most therapeutically-relevant antibodies, in a shorter period of time, with the highest probability of succeeding to clinical trials.
ImmunoPrecise operates from state-of-the-art laboratory facilities located at the Vancouver Island Technology Park in Victoria, British Columbia, in collaboration with its wholly-owned subsidiary operations at U-Protein Express B.V., in the Life Science Incubator, Utrecht, and ModiQuest Research, Oss, both in the Netherlands. The Company operates globally to offer antibody services from target analysis to pre-clinical studies.
The services offered to clients include antibody discovery against a broad spectrum of antigens, including challenging targets. Amongst these services, the Company offers hybridoma production, B-cell services, and a variety of phage display platforms. The Company also provides a broad range of supporting services including immunologically-based assays, recombinant protein manufacturing, humanization, optimization, stable cell line development, and advanced solutions to challenges faced by clients in antibody-related research and development. The antibodies produced by ImmunoPrecise target a wide variety of therapeutic, diagnostic and research applications.
ImmunoPrecise Antibodies Announces DTC Eligibility
VICTORIA, BC, Feb. 28, 2019 /CNW/ - IMMUNOPRECISE ANTIBODIES LTD. (the “Company” or “IPA”) (TSX VENTURE: IPA) (OTC PINK: IPATF) is pleased to announce that its common shares are now eligible for electronic clearing and settlement through the Depository Trust Company (" DTC ") in the United States.
DTC is a subsidiary of the Depository Trust & Clearing Corporation, a U.S. company that manages the electronic clearing and settlement of publicly traded companies. Securities that are eligible to be electronically cleared and settled through DTC are considered to be “DTC eligible”. DTC eligibility is expected to simplify the process of trading and enhance liquidity of the Company’s common shares in the United States.
“The ability to have ImmunoPrecise shares electronically transferred between brokerages in the United States is significantly more convenient and reduces the costs incurred in trading shares,” stated Lisa Hebling, CFO of ImmunoPrecise. “With our shares now traded electronically, existing investors benefit from greater liquidity and execution speed s , while we’ve also opened the door to new investors that may have been previously restricted from our stock.”
IPA est en processus de produire à l’interne ses propres cibles. Fort de ses capacités de production la compagnie va développer un nouveau pipeline de revenu avec royautés attachés par projet. Cette acquisition met en perspective le valorisation de seulement quelques cibles.
Merck to Acquire Immune Design
Acquisition Bolsters Capabilities in Vaccine Development for Infectious Diseases and Cancer
KENILWORTH, N.J. & SEATTLE & SOUTH SAN FRANCISCO, Calif.–(BUSINESS WIRE)-- Merck (MRK), known as MSD outside the United States and Canada, and Immune Design (IMDZ), today announced that the companies have entered into a definitive agreement under which Merck, through a subsidiary, will acquire Immune Design for $5.85 per share in cash for an approximate value of $300 million.
This press release features multimedia. View the full release here:https://www.businesswire.com/news/home/20190221005198/en/
“Scientists at Immune Design have established a unique portfolio of approaches to cancer immunization and adjuvant systems designed to enhance the ability of a vaccine to protect against infection, which could meaningfully improve vaccine development," said Dr. Roger M. Perlmutter, president, Merck Research Laboratories. “This acquisition builds upon Merck’s industry-leading programs that harness the power of the immune system to prevent and treat disease.”
Immune Design is a late-stage immunotherapy company employing next-generation in vivo approaches to enable the body’s immune system to fight disease. The company’s proprietary technologies, GLAAS® and ZVex®, are engineered to activate the immune system’s natural ability to generate and/or expand antigen-specific cytotoxic immune cells to fight cancer and other chronic diseases.
“Merck has a rich history of discovery and innovation and a strong track record of developing meaningful therapeutics and vaccines,” said Dr. Carlos Paya, president and chief executive officer, Immune Design. “We believe this agreement creates shareholder value by positioning our technologies and capabilities for long-term success with a leading, research-driven biopharmaceutical company.”
Under the terms of the acquisition agreement announced today, Merck, through a subsidiary, will initiate a tender offer to acquire all outstanding shares of Immune Design. The closing of the tender offer will be subject to certain conditions, including the tender of shares representing at least a majority of the total number of Immune Design’s outstanding shares, the expiration of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act and other customary conditions. Upon the successful completion of the tender offer, Merck will acquire all shares not acquired in the tender through a second-step merger. The transaction is expected to close early in the second quarter of 2019.
Credit Suisse acted as financial advisor to Merck in this transaction and Gibson, Dunn & Crutcher LLP as its legal advisor. Lazard acted as financial advisor to Immune Design and Cooley LLP as its legal advisor.
Important Information about the Tender Offer
The tender offer described in this press release (the “Offer”) has not yet commenced. This press release is for informational purposes only and is neither an offer to purchase nor a solicitation of an offer to sell any shares of the common stock of Immune Design or any other securities. At the time the planned tender offer is commenced, a tender offer statement on Schedule TO, including an offer to purchase, a letter of transmittal and related documents, will be filed by Merck Sharp & Dohme Corp. (“Merck”) and Cascade Merger Sub Inc., a wholly-owned subsidiary of Merck (“Buyer”), with the Securities and Exchange Commission (the “SEC”), and a solicitation/recommendation statement on Schedule 14D-9 will be filed by Immune Design with the SEC. The offer to purchase shares of Immune Design common stock will only be made pursuant to the offer to purchase, the letter of transmittal and related documents filed as a part of the Schedule TO.
INVESTORS AND SECURITY HOLDERS ARE URGED TO READ BOTH THE TENDER OFFER STATEMENT AND THE SOLICITATION/RECOMMENDATION STATEMENT REGARDING THE OFFER, AS THEY MAY BE AMENDED FROM TIME TO TIME, WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION.
Investors and security holders may obtain a free copy of these statements (when available) and other documents filed with the SEC at the website maintained by the SEC at www.sec.gov or by directing such requests to the Information Agent for the Offer, which will be named in the tender offer statement. Additional copies of the tender offer materials may be obtained at no charge by contacting Merck at 2000 Galloping Hill Road, Kenilworth, N.J., 07033 or by phoning (908) 423-1000. In addition, Merck and Immune Design file annual, quarterly and current reports and other information with the SEC. You may read and copy any reports or other information filed by Merck or Immune Design at the SEC public reference room at 100 F Street, N.E.,Washington, D.C., 20549. For further information on the SEC public reference room, please call 1-800-SEC-0330. Merck’s and Immune Design’s filings with the SEC are also available to the public from commercial document-retrieval services and at the SEC’s website at www.sec.gov.
About Merck
For more than a century, Merck, a leading global biopharmaceutical company known as MSD outside of the United States and Canada, has been inventing for life, bringing forward medicines and vaccines for many of the world’s most challenging diseases. Through our prescription medicines, vaccines, biologic therapies and animal health products, we work with customers and operate in more than 140 countries to deliver innovative health solutions. We also demonstrate our commitment to increasing access to health care through far-reaching policies, programs and partnerships. Today, Merck continues to be at the forefront of research to advance the prevention and treatment of diseases that threaten people and communities around the world - including cancer, cardio-metabolic diseases, emerging animal diseases, Alzheimer’s disease and infectious diseases including HIV and Ebola. For more information, visit www.merck.com and connect with us on Twitter, Facebook, Instagram,YouTube and LinkedIn.
About Immune Design
Immune Design is a late-stage immunotherapy company employing next-generation in vivo approaches to enable the body’s immune system to fight disease. The company’s technologies are engineered to activate the immune system’s natural ability to generate and/or expand antigen-specific cytotoxic immune cells to fight cancer and other chronic diseases. G100, the company’s lead product candidate, is a potent intratumoral TLR4 agonist that has shown clinical benefit in multiple tumor types. Immune Design’s technologies, the fundamental components of which were licensed from the California Institute of Technology and the Infectious Disease Research Institute (IDRI), also have potential application in infectious disease and allergy indications, which are being developed through ongoing pharmaceutical collaborations. Immune Design has offices in Seattle and South San Francisco. For more information, please visit www.immunedesign.com.
ImmunoPrecise Antibodies Reports Record Q3 2019 Financials Results
NEWS PROVIDED BY
ImmunoPrecise Antibodies Ltd.
Mar 27, 2019, 18:26 ET
- Revenues Up 228% For The First 9 Months of Fiscal 2019
VICTORIA, March 27, 2019 /CNW/ - IMMUNOPRECISE ANTIBODIES LTD. (the “Company” or “ImmunoPrecise”) (TSX VENTURE: IPA) (OTC PINK: IPATF) today reports its financial results for Q3 ended January 31, 2019. The financial statements and related management’s discussion and analysis (“MD&A”) can be viewed on SEDAR at www.sedar.com.
Financial Highlights:
Revenue. During the three months ended January 31, 2019, the Company increased revenues to $2,695,583 from $1,723,308 in 2018. This represents a 56% increase in revenue and stems from the acquisitions of U-Protein and ModiQuest, the Company’s ability to grow its core business and expand its market share in Europe, and an increase in projects for the B-cell lab.
Gross Margin. During the three months ended January 31, 2019, the Company increased its gross margin to $1,568,055 from $989,203 in 2018. In percentage terms, the Company’s gross margin increased to 58% from 57% in 2018. The higher gross margin in 2019 was mostly attributable to the fact that the Company focused on higher margin projects at its new B-cell lab and introduced additional efficiencies into its operations. The Company anticipates that gross margin on a percentage basis will continue to be over 50% for the balance of fiscal 2019.
Net Loss. The Company recorded a net loss of $1,187,056 during the three months ended January 31, 2019, which is consistent with the net loss of $1,211,591 for three months ended January 31, 2018. $577,720 of the costs incurred during the three months ended January 31, 2019 were one-time costs which are not expected to be incurred again. These one-time costs included costs incurred to improve operational efficiency across all the divisions, to integrate U-Protein and ModiQuest into IPA’s global network, and to continue to establish a global structure for reporting and oversight. In the current period the Company also made investments that would enable its future growth, such as new management hires, more training programs, and additional space
Adjusted EBITDA. For the three and nine months ended January 31, 2019, excluding the one-time costs of $577,720 and $1,251,613, respectively, EBITDA would have been $116,367 and ($301,477), respectively.
Corporate:
On August 20, 2018, the Company announced that Mr. Guy Champagne resigned from his position as a Director of the Company, and joined the Company’s Advisory Board.
On November 21, 2018, the Company announced that Paul Andreola was appointed as a Director of the Company at its annual general meeting (“AGM”) held in Vancouver, BC on November 20, 2018. Mr. Andreola has over 20 years of business development and financial markets experience including senior management, marketing, and communications roles for early stage companies. Previously in his career, Mr. Andreola was a licensed investment advisor for over 10 years and has facilitated multiple early stage private and public companies in the resource and technology sectors. Mr. Andreola is currently the CEO and Director of NameSilo Technologies Corp. (CSE: URL) and Ironwood Capital Corp. (TSXV: IRN.P).
About ImmunoPrecise Antibodies Ltd.
ImmunoPrecise is a full-service, therapeutic antibody discovery company focused on the next generation of antibody discovery, to deliver the most therapeutically-relevant antibodies, in a shorter period of time, with the highest probability of succeeding to clinical trials.
ImmunoPrecise operates from state-of-the-art laboratory facilities located at the Vancouver Island Technology Park in Victoria, British Columbia, in collaboration with its wholly-owned subsidiary operations at U-Protein Express B.V., in the Life Science Incubator, Utrecht, and ModiQuest Research, Oss, both in the Netherlands. The Company operates globally to offer antibody services from target analysis to pre-clinical studies.
The services offered to clients include antibody discovery against a broad spectrum of antigens, including challenging targets. Amongst these services, the Company offers hybridoma production, B-cell services, and a variety of phage display platforms. The Company also provides a broad range of supporting services including immunologically-based assays, recombinant protein manufacturing, humanization, optimization, stable cell line development, and advanced solutions to challenges faced by clients in antibody-related research and development. The antibodies produced by ImmunoPrecise target a wide variety of therapeutic, diagnostic and research applications.
Forward Looking Information
This news release contains statements that, to the extent they are not recitations of historical fact, may constitute “forward-looking statements” within the meaning of applicable Canadian securities laws. The Company uses words such as “may”, “would”, “could”, “will”, “likely”, “expect”, “believe”, “intend” and similar expressions to identify forward-looking statements. Any such forward-looking statements are based on assumptions and analyses made by ImmunoPrecise in light of its experience and its perception of historical trends, current conditions and expected future developments. However, whether actual results and developments will conform to ImmunoPrecise’s expectations and predictions is subject to any number of risks, assumptions and uncertainties. Many factors could cause ImmunoPrecise’s actual results to differ materially from those expressed or implied by the forward-looking statements contained in this news release. Such factors include, among other things, actual revenues and earnings for IPA being lower than anticipated, and those risks and uncertainties described in ImmunoPrecise’s annual management discussion and analysis for the fiscal year ended April 30, 2017 which can be accessed at www.sedar.com. The “forward-looking statements” contained herein speak only as of the date of this press release and, unless required by applicable law, ImmunoPrecise undertakes no obligation to publicly update or revise such information, whether as a result of new information, future events or otherwise.
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
SOURCE ImmunoPrecise Antibodies Ltd.
For further information: For investor relations please contact: Frederick Chabot, Phone: 1-438-863-7071, Email: frederick@contactfinancial.com; Contact Financial Corp., 1450 - 701 West Georgia St., Vancouver, BC V7Y 1G5
ImmunoPrecise Launches DeepDisplay(™) Antibody Discovery Platform
Canada NewsWire
VICTORIA, April 2, 2019
ImmunoPrecise Launches Large-Scale Discovery Program with US Pharma Company
VICTORIA, April 2, 2019 /CNW/ - IMMUNOPRECISE ANTIBODIES LTD. (the « Company » or « IPA ») (TSX VENTURE: IPA) (OTC PINK: IPATF) announces the expansion of their service offerings in therapeutic antibody discovery to select rare, fully human antibodies using DeepDisplayTM, a combination of transgenic animal immunization and custom phage display antibody selections.

ImmunoPrecise has launched, in response to a large-scale discovery program with an unnamed, US-based Pharma company, a powerful and unique platform for therapeutic antibody discovery proven to be successful in delivering a sequence-diverse human antibody panel with broad species cross-reactivity (e.g. mouse, primate, human). The combination of a transgenic animal immunization with phage display antibody selection used in DeepDisplay™ delivers the most therapeutically-relevant antibodies, in a shorter period of time, with the highest probability of success compared to conventional technologies.
« The ability to select needle-in-the-haystack antibodies using this powerful combination of OmniAb® rodent (mouse or rat) immunization and phage display antibody selections provide an entirely new avenue in therapeutic antibody discovery. » said Debby Kruijsen, Ph.D., General Manager of ModiQuest® Research.
Jennifer Bath, Ph.D., CEO of ImmunoPrecise added: « With over 15 years of experience in customized and precision-based phage display, IPA has coupled this powerful technology with Ligand’s OmniAb™ transgenic rodent platforms to enable the accelerated discovery of highly diverse, fully human antibodies with superior clinical safety and efficacy and the lowest immunogenicity. »
The data generated during the large pharma and OmniAb collaboration will be publicly presented at conferences in Munich, Germany https://www.hub-xchange.com/europe-2019 and Boston, Massachusetts https://www.hub-xchange.com/us-east-coast-2018, in March and May, respectively, as well as via live webinar, details forthcoming.
La compagnie laisse présager que la demande de la part des grosses compagnies pharmaceutiques semble bien réelle :
ImmunoPrecise Antibodies Leverages Proprietary Technology and Expertise to Create Talem Therapeutics
VICTORIA, April 4, 2019 /CNW/ - IMMUNOPRECISE ANTIBODIES LTD. (the “Company” or “IPA”) (TSX VENTURE: IPA) (OTC PINK: IPATF) announces it has formed a wholly-owned, Cambridge, Massachusetts-based subsidiary, Talem Therapeutics (Talem). Talem is focused on the discovery and development of next-generation, fully-human, monoclonal therapeutic antibodies targeting neurology, immuno-oncology, gastroenterology, inflammation, and rare/specialty diseases. Using proprietary antibody discovery platforms and innovative technologies, Talem will be producing a rich pipeline of high quality and distinct antibodies.
The demand for safe, therapeutic, monoclonal antibody (mAb) candidates with more complex target product profiles is driving a need for more robust discoveries. Talem was founded to expedite the high-powered discovery and development of custom therapeutics produced as both internal assets as well as in response to partnership requests with large pharma.
Talem Therapeutics’ strategy is to develop on-demand and in-house antibodies through pre-clinical stage prior to licensing for clinical partners. The speed and adaptability of IPAs platforms enables Talem to quickly respond to targets based on years of experience and expertise in the antibody discovery sector. Along with the designated pre-clinical programs, Talem is also engaging in negotiations on joint research and development (R&D) programs with numerous pharmaceutical companies.
Voici mes notes (brouillon) suite au webcast :
- La compagnie offre une plate-forme de communication accessible en tout temps par le client, cela facilite la communication et permet au client d’être directement impliqué dans le processus
- Selon la CEO, les délais d’exécution pour les mandats seraient environ 3x plus rapide que la compétition actuellement dû au fait qu’ils sont un «one stop shop» et certains compétiteurs utilisent des technologies dépassées
- L’année fiscale se terminant au 18 avril 2019 est sur un run rate de 12M$ de revenus
- Les contrats B-CELL et Custom Phage Display oscillent entre 350k$US et 450k euros – seulement pour la portion initiale de «découverte»
- Marge de profit brute entre 65%-90%
- 1er projet serait en cours dans le laboratoire de Victoria (à vérifier)
- Un client aurait déplacé 13 mandats différents qui avait été attribué anciennement au plus gros compétiteur d’Immunoprecise car meilleur chance de découvrir des candidats potentiels
- Ils sont entrain de bâtir activement leurs actifs de propriété intellectuelle (anticorps?) au site de Victoria ainsi qu’aux Pays Bas et ce depuis les 5 derniers mois
- Le font aussi en partenariat avec des gros joueurs pharmaceutiques
- Les clients paient habituellement 40-60% des coûts avant que les mandats commencent (ça reste reconnu de façon différé dans les états financiers jusqu’à la fin du projet)
- Paiement de royauté, après avoir reçu un «lead candidate» et va suivre jusque dans les études pré-cliniques
- Ils ont lancé des installations pour études pré-clinique au site de Victoria
- Les revenus ont doublé au site de Victoria
- l’offre de service est intéressante pour les clients car ils ont au dessus de 10 variétés d’animaux
- Ils évaluent un uplisting sur le Nasdaq pour 2019 (personnellement je vois mal comment ils pourraient le faire à moins de faire un rollback des actions car il faut minimum 2$/action en US….mais je ne connais pas beaucoup ça)
- Ils auraient déjà reçu de l’intérêt pour se faire acquérir mais la compagnie pense que c’est encore très tôt pour ça. Le but premier n’est pas de se faire acquérir mais si l’opportunité vient éventuellement, ce sera aux actionnaires de décider.
- Intérêt venant d’entreprises GMP (Good Manufacturing Practice)