IPA’s CEO Dr. Jennifer Bath to Appear Live Wednesday on BNN Television
Salut @Frederick_Chabot
Est-ce que Sauvage Publishing est directement lié avec toi et/ou Contact Financial ?https://sauvagepublishing.com/
Combien IPA paye pour ce service? Je vois juste le disclaimer sur CHM (210k$ pour 6 mois de awareness)
C’est ma firme de Marketing Digital. Dans le cas de IPA nous ne produisons que la documentation tel que la présentation corporative, logo design etc, IPA n’a pas engagé Sauvage pour un programme de distribution sur nos liste d’investisseurs.
Merci de la réponse 
D’ici quelques semaines nous allons lancé le site web qui proposera aux investisseurs une plateforme pour démontrer leur talent d’investisseurs. Nous offrirons aux meilleurs la possibilité de publier leur rendement à nos 9M d’investisseurs principalement aux États-Unis. @pbergeronbelanger pourra ainsi démontrer son génie 
IPA Delivers Lead Candidate Antibodies from B Cell Select™ Platform for at Home SARS-CoV-2 Diagnostic Test
Also signs Definitive Commercial Agreement with University of Victoria
VICTORIA, BC, Oct. 8, 2020 /CNW/ - IMMUNOPRECISE ANTIBODIES LTD. (the « Company » or « IPA ») (TSXV: IPA) (OTCQB: IPATF) (FSE: TQB2), a leader in full-service, therapeutic antibody discovery and development, today announced an update on their NSERC-funded partnership with the University of Victoria for the generation of a real-time cell-phone based COVID-19 antibody diagnostic test.
In May, the Company announced the partnership objective to develop a colorimetric, easy to use, saliva test for the detection and screening of SARS-CoV-2. The test is designed to have a detectable color change that can be easily measured using a cell phone camera and application. The results, including the general location (by zone rather than by specific household, to comply with privacy rights) of the test, can be uploaded to a cloud-hosted database. This will provide immediate information about the number of infections and the general locations to the health authorities.
Dr. Alexandre Brolo’s lab at the University of Victoria has succeeded in optimizing the chemistry to be used in a critical part of the diagnostic application- the consistent modification of strips with nanoparticles that present a distinct color change upon the binding of antibodies to SARS-CoV-2 proteins.
As an additional milestone in the development of the diagnostic, Dr. Brolo’s research team, well known for their historical work in the development of diagnostics for the Zika virus, has designed and 3D-printed a reader prototype enabling the use of the diagnostic application within a standard mobile phone.
As a prototype proof of concept, the research team at the University of Victoria has demonstrated significant and detectable color change when testing the use of the diagnostic with a commercial mobile phone app. The test has been further validated using an assay demonstrating the flow of validated human IgG antibody samples across the surface of the nanoparticle-coated strip containing SARS-CoV-2 antigen.
Antibodies developed for diagnostic purposes require broad epitope coverage, high specificity, low background noise and heightened affinity. Monoclonal antibodies with these characteristics can detect nuanced differences between similar molecules more readily, to avoid false positive results.
The rabbit’s immune system is unique in that it creates highly diverse repertoire of antibodies via its unique gene conversion mechanism and the antibodies themselves have more variation in length and sequence compared to rodents and humans. Rabbit antibodies can identify epitopes on molecules that are non-immunogenic in other species.
The combination of ImmunoPrecise’s B cell Select™ platform and the rabbit’s immune system produces antibodies particularly useful for applications that require extremely low background to allow for the detection and verification of low-level biomarkers with little to no-cross reactivity. IPA has performed over 150 successful diagnostic rabbit B cell programs, many of which have been for point of care lateral flow assays.
« Rabbit monoclonal antibodies developed via our B cell Select™ platform consistently provide panels of highly specific, non-cross-reactive antibodies which are adaptable to any immunodiagnostic format, » states Dr. Andra Li, Scientific Director and co-developer of B cell Select™.
Upon successful commercialization of the diagnostic kit, ImmunoPrecise has committed to paying 5% of Global Net Revenue to the University of Victoria.
Wow! ça c’est cool.
U.S. aims to get 1M doses of COVID antibody before 2021
“The interest in the monoclonal antibodies is quite high,” said Janet Woodcock, director of the Center for Drug Evaluation and Research at the FDA. Woodcock, who is serving as a part of Operation Warp Speed, has recused herself from taking part in the approval decisions related to coronavirus therapeutics.
Entrevue de Dr Jennifer Bath sur Fox Business News (16 Oct)
(https://video.foxbusiness.com/v/6201838860001/#sp=show-clips)
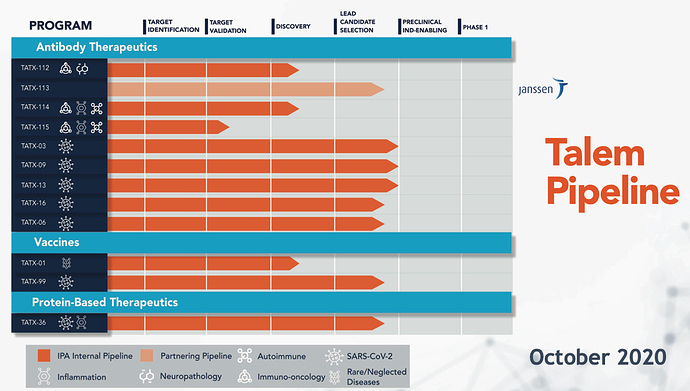
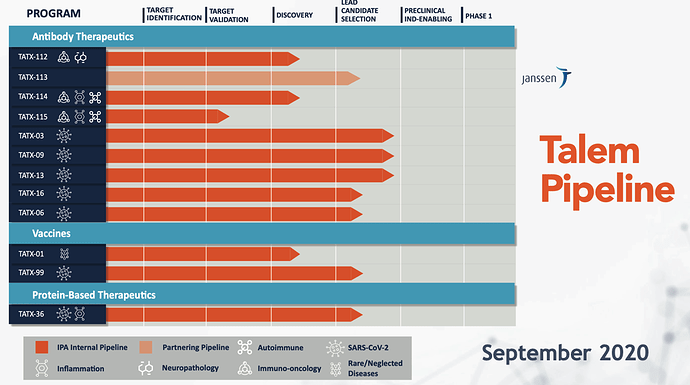
Image de la nouvelle présentation aux investisseurs (octobre 2020). Les programmes TATX-99 (Vaccines) et TATX-36 (protein-based therapeutics) se rapprochent des essais pré-cliniques. (voir l’avancement vs la présentation aux investisseurs de septembre 2020)
De même que les programmes thérapeutiques par ancticorps TAXT-113, TAXT-16 et TAXT-06.
Immunoprecise mentionné dans un article de CBC
A closer look at Canada’s homegrown COVID-19 vaccine candidates
Immunoprecise Antibodies
Location: Victoria, B.C.
Vaccine type: Protein subunit
Stage of development: Preclinical
The Immunoprecise Antibodies vaccine is a protein-based vaccine, a relatively traditional and widely used vaccine type, where pieces of viral protein are injected to teach the immune system to recognize them. (Among the international frontrunners using this technique is Novavax, which is in Phase 3 clinical trials)
As its name suggests, Immunoprecise Antibodies’s main specialization isn’t vaccines but developing antibody-based therapies. The company has been working on treatments for COVID-19 looking at exactly what kind of antibodies in humans and other animals bind to the SARS-CoV-2 spike protein and inactivate or neutralize it. Those antibodies could potentially be mass-produced as a therapy or treatment.
The researchers need to know what specific part of the spike protein each antibody binds to so they can target different parts of it. But knowing that also allows them to « reverse engineer » those fragments of the spike protein to create a vaccine in parallel with the therapeutics.
Jennifer Bath, the company’s president and CEO, acknowledges it’s an approach that takes longer than the strategies used by some of the frontrunning vaccines, which typically target the entire spike protein or the so-called receptor binding domain (RBD).
But she says targeting fragments known to lead to a protective immune response reduces the chance that some parts of the protein will trigger the immune system in a dangerous way, as can happen during a real COVID-19 infection.
« The more you can reduce that down to only the portions that are really important for providing immunity … the less adverse events you’re likely to have. »
While Immunoprecise Antibodies hasn’t itself produced a vaccine before, Bath and some other colleagues have previously used this technique to design other types of vaccines while working at other companies.
The vaccine currently includes three spike protein fragments produced using genes inserted into mammalian cells. It also includes an adjuvant from Netherlands-based LiteVax. In September, the vaccine started preclinical trials on pigs in Spain, funded by the European Commission’s TRANSVAC2 vaccine development network. Results are expected at the end of the year, and the company hopes to start clinical trials next year following preclinical trials on monkeys.
@MaxVegan, bienvenu sur EMC. La communauté ici identifie et discute de titre intéressants qui ont parfois très bien performés ainsi que d’autres qui ont fait moins bien (i.e mouvement de côté pendant des mois) et d’autres qui sont littéralement passés au tordeur. Il est donc important de modérer ses ardeurs quand on investit dans les microcaps, diversifier et être prêt à voir certains titres reculer de 50%, 90% voir faire faillite. Alors il est important de ne pas mettre tout ces oeufs dans le même panier. Mon conseil de bienvenu en ce vendredi d’automne.
Non, y a pas juste des winners (titres) ici. Juste une bonne communauté qui partage des idées d’investissement tout en élaborant /validant les thèses d’investissement au fils des semaines, mois et années. Je suis à peu près certain que la majorité ici a reçu son lot de claques au fil des ans alors attends toi au même processus « d’apprentissage » même en procédant avec prudence. Bref, si t’es nouveau au monde des microcaps mieux vaut commencer avec de petits pas plutôt que d’essayer de courir en sortant des blocs. Bonne chance.