Une de mes positions $ATE.V Antibe Therapeutics a reçue l’approbation de Santé Canada pour procéder à la phase II du développement de leur drogue ATB-346, un anti-inflammatoire NSAID sans les effets gastriques des médicaments tel l’ibuprofen. http://www.antibethera.com/2016/03/07/antibe-therapeutics-receives-approval-to-proceed-to-phase-2-clinical-trial/
La compagnie a aussi signé une entente avec Knight Therapeutics $GUD.TO pour les droits de commercialisation: http://www.antibethera.com/2015/11/16/antibe-enters-into-a-gud-product-licensing-agreement-with-knight-therapeutics/
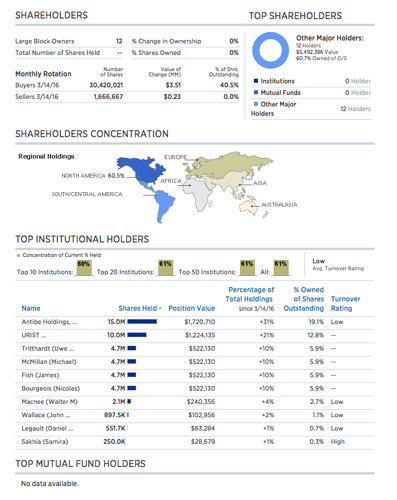
L’ex CFO de Paladin Labs Samrira Sakhia est sur le board:
Le CEO de Knight Therapeutics Jonathan Goodman était sur le board de la compagnie et détient encore des actions dans $ATE.V et via la placement de Bloom Burton Healthcare fund.
“The world needs safer anti-inflammatory drugs, and we are pleased to have secured Antibe’s innovative portfolio for our territories. We have come to know their management team and are also pleased to provide financial support for their strategic thrust into regenerative medicine”, said Jonathan Ross Goodman, President and CEO of Knight.
Antibe a fait l’acquisition de la Compagnie Citagenix qui les supportes comme réseau de vente et distribution qui a un estimé de revenu de $10mil par année.
Les états financiers au Q4 2015 avec les revenus de Citagenix sont disponibles sur Sedar. http://www.antibethera.com/2015/10/06/antibe-therapeutics-announces-strategic-transactions/
Pipeline de drogue: http://www.antibethera.com/pipeline/
60% de actions est détenues par des insider:
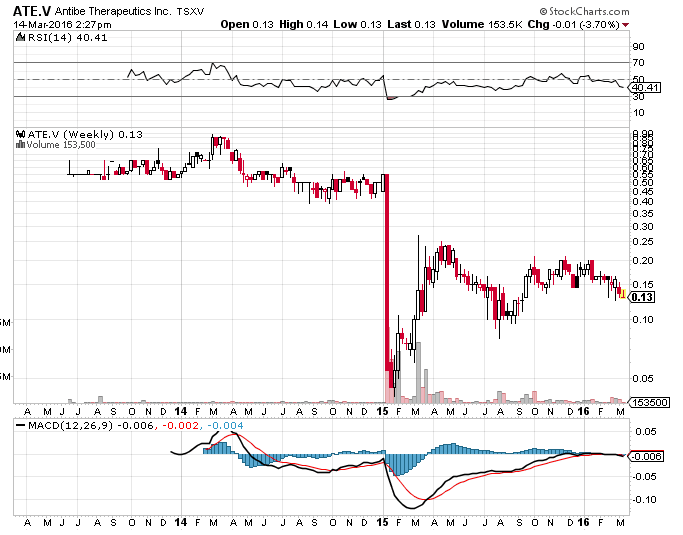
Voici la charte. L’action avait été démolie en janvier 2015 suite aux résultats de la phase I qui demandait plus de validation des tests:
Selon le MD&A la compagnie veut amener leur drogue ATB-346 jusqu’à la fin de la phase II avec une sortie stratégique.
Personnellement, je trouve intéressant le network et les personnes impliqués dans cette compagnie et le progrès qu’ils ont fait depuis le résultat. Je suis long. Mes premières actions ont été acheté à $0,07 jusqu’à $0.15.
Le site web de la compagnie:
http://www.antibethera.com